ISSN-Online: 1819-6187; Frequency: Annual, Instant published;The first Issue: June, 2013; Full Open Access; Publishing fee
SCOPE: The journal publishes original research, reviews and other material related to breast cancer, gastrointestinal cancer, hematologic malignancies, molecular oncology, lung cancer, genitourinary cancer, head and neck cancer, pediatric oncology, neurooncology, supportive care and quality of life issues, prevention, and phase I and clinical pharmacology. The journal publishes research papers in areas of research include, but are not limited to, the following: Clinical diagnosis, laboratory diagnosis, differential diagnosis, imaging tests, pathological diagnosis, molecular biological diagnosis, immunological diagnosis, genetic diagnosis, functional diagnostics, and physical diagnosis; and comprehensive therapy, drug therapy, surgical therapy, interventional treatment, minimally invasive therapy, and robot-assisted therapy.
MISSION: The mission of the journal is the rapid exchange of scientific information between clinicians and scientists worldwide, seeks to publish high-quality practical and clinical research around new and existing therapies in all areas of cardiology. The mission of the journal is the rapid exchange of scientific information between clinicians and scientists worldwide.
Editor In Chief

Yoshihito Yokoyama, born on January 25, 1963. He has been Associate Professor at the Department of Obstetrics and Gynecology, Hirosaki University Graduate School of Medicine, Aomori, Japan.
MoreCover Image
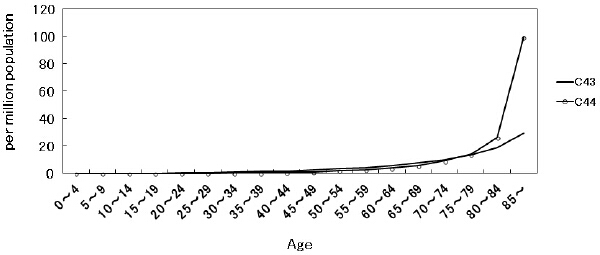
Figure 1 Crude mortality rates of C43 and C44 (from 1999 through 2014) by 5-year age groups.
MoreFeatured
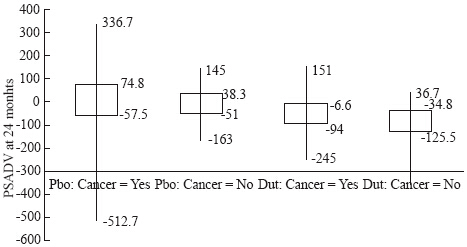 AIM: Prostate specific antigen (PSA) is a widely utilized screening marker for prostate cancer. Its performance in detecting prostate cancer is enhanced with the 5α-reductase inhibitor (5aRI) dutasteride. We evaluated if PSA density velocity (PSADV) further improved the ability to predict prostate cancer risk on repeat biopsy utilizing the REDUCE trial data in order to avoid unnecessary biopsies. MATERIALS AND METHODS: The REDUCE study randomized 8 231 men aged 50 to 75 years with a PSA between 2.5 and 10 ng/mL and a previously benign prostate biopsy. Prostate volume, PSA and biopsy results at 24 months were available for 1 074 subjects. PSADV, defined as the change in PSA density divided by the number of days between PSA measurements (ng/mL/cc/day), was calculated and compared between the placebo and treatment groups and further stratified by prostate biopsy results. Statistical significance was calculated using the Wilcoxon rank sum test.More
AIM: Prostate specific antigen (PSA) is a widely utilized screening marker for prostate cancer. Its performance in detecting prostate cancer is enhanced with the 5α-reductase inhibitor (5aRI) dutasteride. We evaluated if PSA density velocity (PSADV) further improved the ability to predict prostate cancer risk on repeat biopsy utilizing the REDUCE trial data in order to avoid unnecessary biopsies. MATERIALS AND METHODS: The REDUCE study randomized 8 231 men aged 50 to 75 years with a PSA between 2.5 and 10 ng/mL and a previously benign prostate biopsy. Prostate volume, PSA and biopsy results at 24 months were available for 1 074 subjects. PSADV, defined as the change in PSA density divided by the number of days between PSA measurements (ng/mL/cc/day), was calculated and compared between the placebo and treatment groups and further stratified by prostate biopsy results. Statistical significance was calculated using the Wilcoxon rank sum test.More
Current
EXCELLENT ARTICLES
Table of Contents
Editorial
| Definition and Staging of Early Esophageal, Gastric and Colorectal Cancer |
PDF
HTML
|
|
Nikolas Eleftheriadis, Haruhiro Inoue, Haruo Ikeda, Manabu Onimaru, Akira Yoshida, Roberta Maselli, Grace Santi, Shin-ei Kudo |
161-178 |
Topic Highlight
Review
| Management of Advanced Medullary Thyroid Carcinoma |
PDF
HTML
|
|
Melvil Šabani, Drago B Jelovac, Milan B Petrović, Miodrag Gavrić |
202-207 |
| Solitary Lung Nodule: The Impact of Computed Tomography on Pre-Test Probability of Malignancy, Lung Cancer Staging And Management |
PDF
HTML
|
|
Aldo Pezzuto, Raffaele Ratta, Yuri Errante, Carlo Cosimo Quattrocchi, Anna Maria Frezza, Pierfilippo Crucitti, Giuseppe Tonini |
129-135 |
Original Article
| Outcomes of Self-Expandable Metallic Stent Insertion on Acute Colorectal Obstruction: A Single Endoscopist Experience |
PDF
HTML
|
|
Vitoon Chinswangwatanakul, Chotirot Angkurawaranon, Chainarong Phalanusitthepha, Asada Methasate, Atthaphorn Trakarnsanga, Jirawat Swangsri, Thawatchai Akaraviputh |
237-240 |
| Comparison of Dose Distribution between Intensity Modulated Radiation Therapy and Dynamic Arc Therapy in and out-of- Field for Prostate Cancer Treatment |
PDF
HTML
|
|
Aymen Ben Abdennebi, Guillaume Auzac, Jean Chavaudra, Mounir Besbes, Damien Llanas, Rodrigue Allodji, Tao Yun Gan, Pierre Blanchard, Attila Veres, André Bridier, Dimitri Lefkopoulos, Florent De Vathaire, Ibrahima Diallo |
|
ISSN: 1819-6187
 Yoshihito Yokoyama, born on January 25, 1963. He has been Associate Professor at the Department of Obstetrics and Gynecology, Hirosaki University Graduate School of Medicine, Aomori, Japan.More
Yoshihito Yokoyama, born on January 25, 1963. He has been Associate Professor at the Department of Obstetrics and Gynecology, Hirosaki University Graduate School of Medicine, Aomori, Japan.More Figure 1 Crude mortality rates of C43 and C44 (from 1999 through 2014) by 5-year age groups. More
Figure 1 Crude mortality rates of C43 and C44 (from 1999 through 2014) by 5-year age groups. More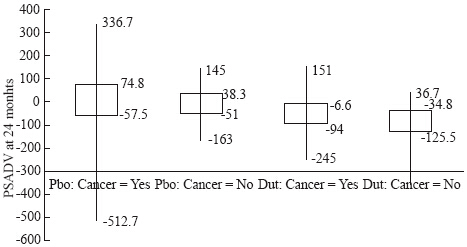 AIM: Prostate specific antigen (PSA) is a widely utilized screening marker for prostate cancer. Its performance in detecting prostate cancer is enhanced with the 5α-reductase inhibitor (5aRI) dutasteride. We evaluated if PSA density velocity (PSADV) further improved the ability to predict prostate cancer risk on repeat biopsy utilizing the REDUCE trial data in order to avoid unnecessary biopsies. MATERIALS AND METHODS: The REDUCE study randomized 8 231 men aged 50 to 75 years with a PSA between 2.5 and 10 ng/mL and a previously benign prostate biopsy. Prostate volume, PSA and biopsy results at 24 months were available for 1 074 subjects. PSADV, defined as the change in PSA density divided by the number of days between PSA measurements (ng/mL/cc/day), was calculated and compared between the placebo and treatment groups and further stratified by prostate biopsy results. Statistical significance was calculated using the Wilcoxon rank sum test.More
AIM: Prostate specific antigen (PSA) is a widely utilized screening marker for prostate cancer. Its performance in detecting prostate cancer is enhanced with the 5α-reductase inhibitor (5aRI) dutasteride. We evaluated if PSA density velocity (PSADV) further improved the ability to predict prostate cancer risk on repeat biopsy utilizing the REDUCE trial data in order to avoid unnecessary biopsies. MATERIALS AND METHODS: The REDUCE study randomized 8 231 men aged 50 to 75 years with a PSA between 2.5 and 10 ng/mL and a previously benign prostate biopsy. Prostate volume, PSA and biopsy results at 24 months were available for 1 074 subjects. PSADV, defined as the change in PSA density divided by the number of days between PSA measurements (ng/mL/cc/day), was calculated and compared between the placebo and treatment groups and further stratified by prostate biopsy results. Statistical significance was calculated using the Wilcoxon rank sum test.More 


